Plasma-enhanced catalysis
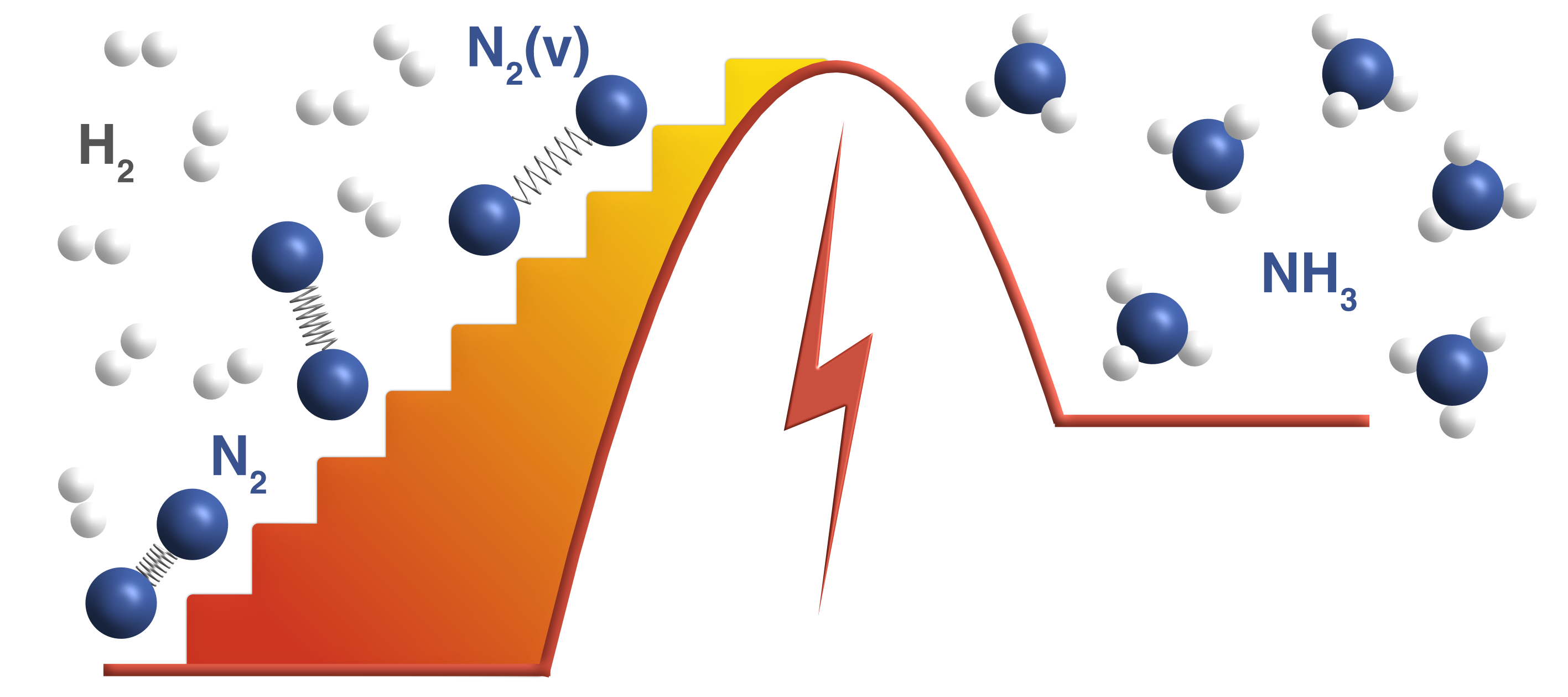
Fritz Haber and Carl Bosch's invention of the ammonia synthesis process is considered to be one of the most important inventions of the twentieth century. Ammonia is used to make inorganic fertilizers and is responsible for feeding about half the world's population. The Haber-Bosch process is typically carried out on the surfaces of solid materials known as catalysts. Even with the best-known catalysts, the process is very energy intensive, and requires very high pressures (100-200 atm) and temperatures (700-800 K) to be feasible. Reducing these temperature and pressure requirements is a grand challenge for sustainable ammonia synthesis.
The central idea behind this research is to assist conventional catalysts (metal nanoparticles on metal oxide supports) by applying an electric discharge (also referred to as a non-thermal plasma). We postulate the plasma can selectively activate the strong triple bond of the nitrogen molecule by vibrational excitation in a way that is not possible by thermal heating in conventional catalysis. I have developed a kinetic model based on quantum mechanical density functional theory calculations that incorporates the effect of N2 vibrational excitation as observed in a non-thermal plasma. Two key insights emerge from this model. The first is that ammonia synthesis rates in the presence of the plasma are expected to be greatly enhanced over thermal rates for a given bulk temperature and pressure. Second, the optimal catalyst materials and active sites in the presence of plasma excitation may not be the same as those for thermal catalysis. Experimental rate measurements by our collaborators confirm that ammonia is produced over metal catalysts at conditions far removed from Haber-Bosch, and the relative trends in activity are found to be consistent with the predicted trends. The work is among the first examples of the computationally guided design of plasma-catalyst systems.
Related Publications
- P.Mehta, P.Barboun, F.Herrera, J.Kim, P.Rumbach, D.B.Go, J.C.Hicks, W.F. Schneider, Overcoming Ammonia Synthesis Scaling Relations with Plasma-enabled Catalysis. Nature Catalysis, 2018, 1, 269-277, 2018. DOI: 10.1038/s41929-018-0045-1 Supporting Dataset:
Editorial Highlight
- F.A. Herrera, G. Brown, P. Barboun, N. Turan, P. Mehta, W.F. Schneider, J.C. Hicks D.B. Go, The Impact of Transition Metal Catalysts on Macroscopic Dielectric Barrier Discharge (DBD) Characteristics in an Ammonia Synthesis Plasma Catalysis Reactor. Journal of Physics D: Applied Physics, 2019, 52, 224002 DOI: 10.1088/1361-6463/ab0c58
- P. Mehta, P. Barboun, D.B. Go, J.C. Hicks, W.F. Schneider, Catalysis Enabled by Plasma Activation of Strong Chemical Bonds: a Review. ACS Energy Letters, 2019, 4, 1115 DOI: 10.1021/acsenergylett.9b00263 ACS Editors' Choice PDF Energy Selects Highlight
- P. Barboun, P. Mehta, F. Herrera, D.B. Go, W.F. Schneider, J.C. Hicks, Distinguishing Plasma Contributions to Catalyst Performance in Plasma-Assisted Ammonia Synthesis. ACS Sustainable Chemistry & Engineering, 2019, 7, 8621 DOI: 10.1021/acssuschemeng.9b00406
Related Cover Art

Related Poster
Comments
Comments powered by Disqus